Daylesford, Victoria, Australia
Geological and mining
history, including the age of volcanoes to the north and south, but not yet of Wombat Hill itself.
This is one of several pages
concerning Daylesford and places nearby. The index for those
pages is here: ../.
2015-10-18 Robin Whittle
rw@firstpr.com.au
Minor updates 2016-01-25, 2016-02-29, 2016-12-05, 2017-07-13 and 2017-12-29.

Daylesford with Wombat Hill Botanical Gardens, looking north-west
from Wheelers Hill Road, 8th August 2014. (Wheelers Hill is another volcano.)
Wombat Hill is a scoria cone volcano - one of the hundreds of
volcanoes of various types in the Newer Volcanic
Province, which stretches to Mt Gambier. (A map here of the whole province; DEPI
map; highly
informative poster.)
My cell-phone's GPS function tells me that the elevation of Wombat Hill
is 667 metres (2,182 feet). By comparison, Mt Dandenong - the
most prominent feature in the horizon around Melbourne - is 633 metres
(2,077 feet). The Wikipedia page for Daylesford https://en.wikipedia.org/wiki/Daylesford,_Victoria
mentions an elevation of 616 metres (2,021 feet). I guess this
would be at the post office. My GPS reports an elevation of 523
metres (1,716 feet) for the walking track close to the dam
of Daylesford Lake.
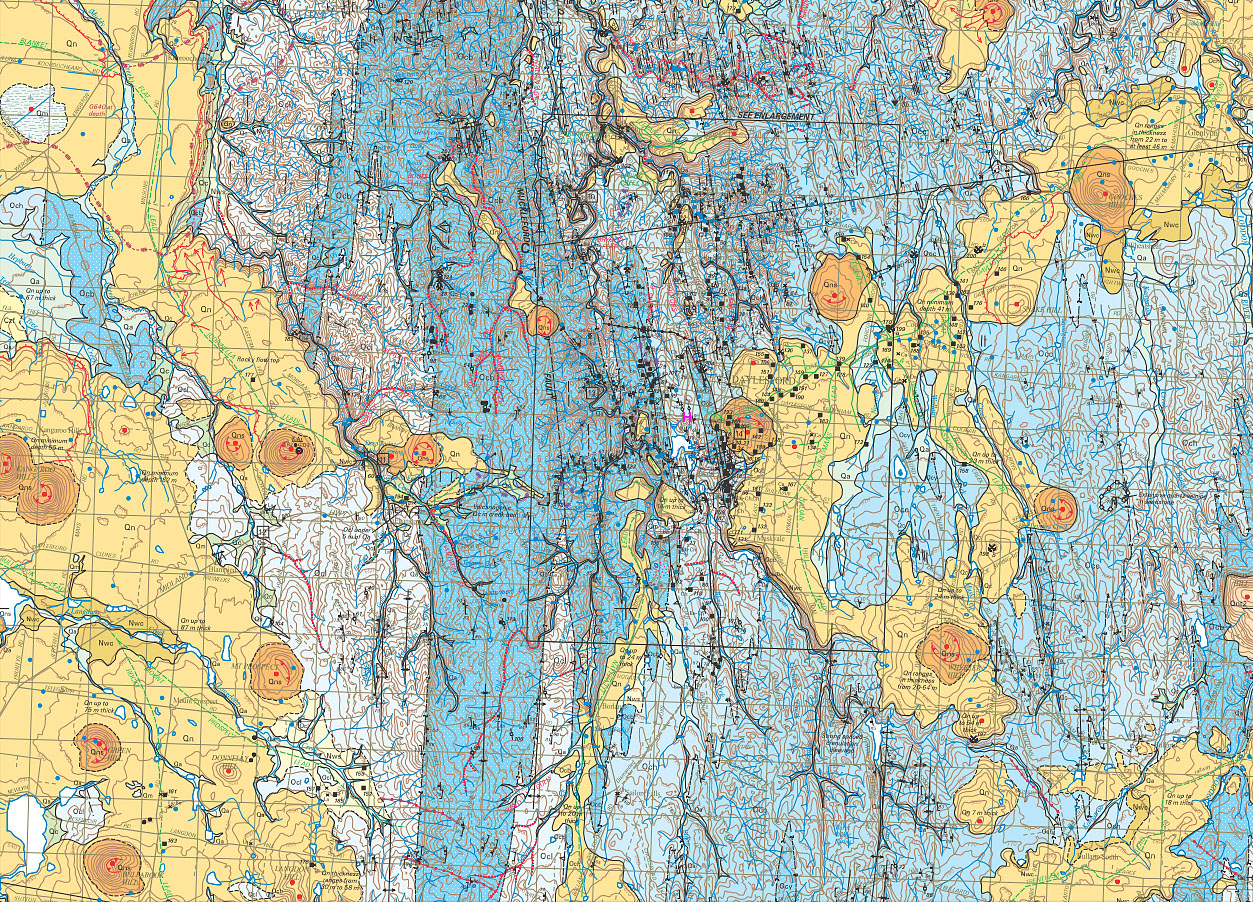
There are numerous volcanoes in the region. These can
be seen as
the orange circular areas in this map, which is freely available from http://dpistore.efirst.com.au/product.asp?pID=292&cID=30&c=125213
. I count 16 volcanoes in this area, which is about 25km west to
east.
To aid with printing the above section of the map, here it is as pages 1 and 2.
If it wasn't for the volcano, the
Daylesford area would be
undulating sedimentary ground, somewhat lower. This rock is
from the trilobite days of the
Lower Ordovician - around 480 million years ago.
The volcanos are much more recent than this - the last few million
years or so. The July 2016 update of the Goldsfields Track Walking and Cycling Guide
(http://www.goldfieldstrack.com.au
page 15) states:
In
this part of the world the youngest volcanoes are 0.5 to 1 million
years old - so young that they still retain their volcanic shape.
Update July 2017:
I tried to find the age of the Wombat Hill volcano but have found any definitive research. The closest I have come is this article:
Ismail-2013
Rafika Ismail, David Phillips &
William D. Birch
40Ar/39Ar dating
of alkali feldspar megacrysts from selected young volcanoes of the
Newer Volcanic Province, Victoria.
Proceedings of the Royal Society of
Victoria 125(1/2): 59–69.
http://www.publish.csiro.au/RS/RS13019
(Open access.)
|
Basalt from the Daylesford Ridge Road quarry: about 2.01 million years ago.
Mt Franklin 8.5km north of Daylesford,
is much more recent: about 110,000 years ago.
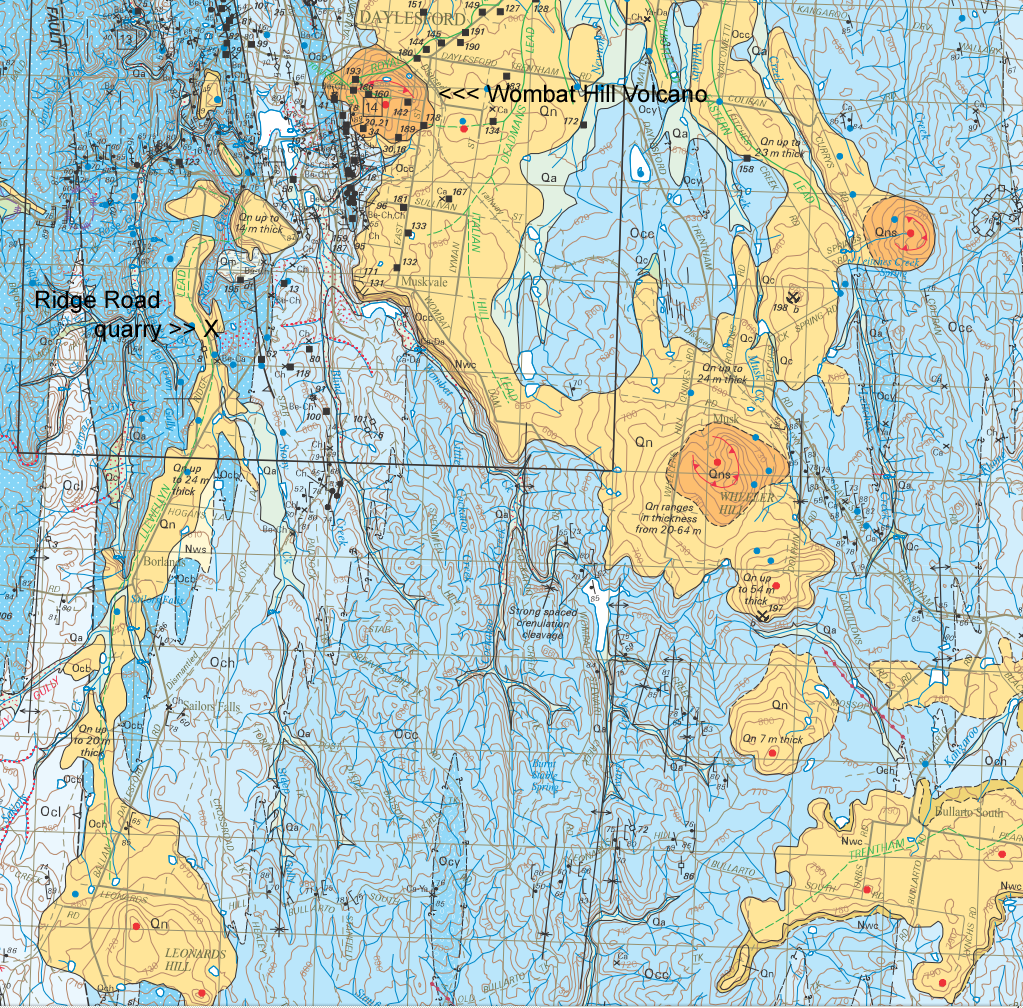
The article refers to the date of a rock sample from a basaltic flow
which (as far as I can see) is from the Leonards Hill volcano, about
9km to the south. Here is a detail from the above map with the
Ridge Road quarry location shown. So we have very different dates
for the two volcanos to the north and south and no date for Wombat Hill
itself.
Here is my understanding of the argon-argon dating
techniques (WP link)
technique used to estimate these ages:
The
researchers find grains of feldspar, or some other suitable crystal
which contains significant amounts or potassium. In the case of
the Daylesford sample, this was a 2cm long feldspar crystal embedded in
basalt from the old quarry on Ridge Road at the corner of Stony Creek
Road, 3km south-south-west of Daylesford (Google
Maps). From the above article [Ismail-2016]:
The
quarry on Ridge Road . . . is in a basaltic
lava flow along the former valley
of Stony Creek,
which is now entrenched along one edge of the
flow. The feldspar sample selected for the present study
consisted of a 2-cm long colourless,
compositionally zoned crystal
fragment enclosed in the basalt.
There are three naturally occurring isotopes of potassium 39K, 40K
and 41K,
with 20, 21 and 22 neutrons respectively in their nuclei. All
have 19 protons, which is what defines these nuclei as being of
potassium atoms, since the 19 protons attract 19 electrons to form an
electrically neutral atom. The outermost electron of a neutral
potassium atom is in its own "shell" (really a cloud of probability
where the electron might be at any moment, if we think of it as a
little ball, which is not quite the whole story . . .). The shell
below is full, with 8 electrons. The lone outer electron is
unstable and the total energy of the atom is significantly reduced if
it can get rid of the electron. So potassium in compounds does
this, and becomes a positively charged ion. In potassium
chloride, the chlorine atoms have seven electrons in their outer
"shell", which is also in a high-energy unstable state. They
accept the electrons from the potassium atoms and so too have full,
low-energy, totally self-satisfied outer electron "shells". The
negatively charged chlorine ions are attracted to the positively
charged potassium ions and form a neat cubic crystal. Sodium
chloride - table salt - is the same.
The number of neutrons in the nucleus of an atom does not affect how
the atom forms compounds, so all three potassium isotopes are
chemically indistinguishable. All three had their nuclei formed
by the extreme pressures and temperatures inside a supernova explosion
of a star - one or more stars whose supernovae debris formed the cloud
from which the Solar System formed about 4.6 billion years ago.
No-one knows how long ago these supernovae were, but these potassium
nuclei have been unchanged since they were first formed by lighter
nuclei being forced together in the supernova, so forcibly that their
mutual repulsion was overcome and they stuck together as new, larger,
nuclei.
39K and 41K
are perfectly stable. However, 40K is unstable AKA radioactive. 0.012% of
potassium in and on the Earth is 40K.
(Wikipedia link) This has a half life of 1.251 billion years, so after this amount of time, half the atoms
of 40K
in a sample would be gone, since their nuclei will have broken down
(decayed) into the nuclei of isotopes of the metal calcium and the
noble gas argon. 10.72% of these decays involve an inner electron
from the atom entering the nucleus and converting one of the protons
into a neutron, with the release of electromagnetic energy as a gamma
ray and a neutrino - an enigmatic particle which can usually pass right
through the Earth without hitting anything!
So in any one year, there is a small, but precisely known, chance that
a 40K
nucleus will transform itself from having
21 neutrons and 19 protons into having 22 neutrons and 18 protons,
which means it is no longer a potassium nucleus, but the nucleus of the
noble gas argon - specifically the stable (non-radioactive) isotope 40Ar.
Since it now has one less positive charge, the nucleus only attracts 18
electrons to form an electrically neutral atom. This is argon,
and its outer electron shell is completely self-satisfied with 8
electrons - the whole atom is in a low energy state and there isn't any
addition or subtraction of electrons which would cause it to be in a
still lower energy state.
This means that argon does not bond covalently (electron sharing) or
bionically (as with sodium and potassium, by giving up or gaining and
electron) with any other atoms. Argon, along with neon and
krypton, are noble gasses which exist in the air we breath. By
mass 0.934% of air is argon, and 99.6% of this is 40Ar
produced, as just described, by the decay of 40K in rocks, magma etc. within the whole Earth.
(There used to be a lot more 40K
when the Earth was first formed. It used to be the most important
source of radioactive heating of the Earth's core and mantle, but it is
now the third-most important after isotopes of uranium and thorium.)
If the feldspar crystal is hot enough, the argon atom behaves as a gas
and can work its way through the crystal structure and so get out of
the whole crystal. However, this does not occur if the crystal is
cool enough.
Long ago - I guess millions to a few billion years ago - a
crystal of feldspar formed, incorporating potassium atoms whose nuclei
were unchanged since being forged in one or more supernovae over 4.5
billion years ago. This crystal somehow got mixed up in a flow of
magma under the Australian continent, where this magma, later called
lava as it erupts from what is now Leonards Hill, is hot enough to be
molten, but not hot enough to melt the feldspar crystal. This
high temperature is, however, enough to allow all the 40Ar atoms which result from the
radioactive decay of 40K
to escape the crystal, go into the magma/lava, and, as the lava flows
from the volcano, escape into the atmosphere.
The freshly erupted lava, with this feldspar crystal in its mist,
flowed down the edge of the volcano, and eventually comes to rest 3.3km
away, where it cools and solidifies, forming the basalt at the quarry
at the corner of Ridge and Stony Creek Roads. The lava
would have cooled within days or months to the point where the
continually produced 40Ar
atoms remain trapped in the crystal.
Because enquiring minds want to know, Rafika Ismail and colleagues went
looking for such a crystal, found it, and took it back to the lab for
analysis. By measuring how many 40Ar atoms are trapped in the crystal, and by
determining (indirectly) how many 40K
atoms are in the crystal now (or were in the past few million years,
since their numbers are declining due to radioactive decay) it is
possible to calculate how long ago the crystal and its surrounding lava
cooled - so giving the age of the presumably single phase of eruptions
which produced Leonards Hill.
This was done by sending the crystal
to a Canadian nuclear reactor facility, where it was bombarded by
neutrons, which converted some proportion of the stable 39K nuclei into radioactive 39Ar
nuclei. (The high speed neutron boots out a proton and takes its
place.)
The exact proportion of this conversion is measured by analysing
another sample which is subject to the same neutron bombardment.
Then the crystal is crushed into small grains and placed in a mass
spectrometer specifically designed for five of the isotopes of argon: the Argus
IV mass
spectrometer at the University of Melbourne, School of Earth Sciences (link).
An infra-red laser heats the grains so that various gases, including
all the argon atoms of various isotopes, escape the crystal. (The
potassium atoms stay behind, ionically bound, since the temperature is
not enough to free them from their ionic bonds with neighboring atoms.)
The argon atoms are then ionised (an electron is added or
removed). These individual ions have the same electric charge,
but different masses, according to the total number of protons and
neutrons in their nuclei. (The electrons are about 1/1,837 the
mass of protons and neutrons, so they hardly contribute little to the
total mass.)
The argon ions are accelerated electrically in a vacuum to form a
narrow beam. The beam is processed by a velocity selector (link)
so that all the ions which emerge have the same velocity.
The beam is bent about 90 degrees by a large electromagnet. The
bending force is the same for all the ions, since they have the same
electrical charge. However, they have different momenta in the
original direction, since they are traveling at the same speed but have
different masses. The heavy ions are bent less than the light
ion, so five separate beams emerge from the magnetic field, one for
each of the five argon isotopes. The number of ions in each of
the five beams is measured simultaneously by the ions coming to a halt,
with their electrical charges adding up to an electrical current, on
five metal plates connected to five very sensitive amplifiers.
The 39Ar
nuclei can only have come from the conversion of neutron beam
conversion of 39K
nuclei. Any such 39Ar
which might have been present in the crystal from millions of years ago
would by now have disappeared, since it has a half-life of 269
years. The count of 39Ar can be used to calculate the crystal's 39K
content, from which its much smaller present day and past (since it is
slowly diminishing) 40K content can then be calculated.
With care, the number of 40Ar
ions detected by the mass spectrometer reflects exactly what was in the
crystal. The crystal has been imbedded in solid, cool, basalt
(through which argon atoms cannot move) since the lava flow
cooled. Ideally, the sample and the spectrometer will not contain
any stray 40Ar
atoms from the atmosphere.
With this simultaneous measurement of the crystal's 40Ar and 39Ar
content, the amount of 40K
(the original radioactive potassium isotope, created in a supernova)
can be calculated for the exact same grains and laser heated gas
release temperatures as are used for the measurement of the
amount of 40Ar
in the crystal (the decay product of this, collected in the years
since the crystal and its surrounding lava cooled). This
overcomes various errors which would result from trying to measure the
potassium and 40Ar
content separately. This reduction of error makes it possible to
calculate dates when even when only a very small fraction of the 40K has decayed since the crystal
cooled enough to trap all the 40Ar.
"Small fraction" in this case means a small fraction of a billion years
or so. So the technique is good for measuring the last melting
time of rocks in the million to billion year range. However, as
far as I know, it is not the best method of measuring much shorter
times, such as of a few thousand years since the Mt Gambier or Mt
Napier eruptions.
Plugging these two measurements, together with a measurement from the
reference sample from the neutron, provides the time since the
cooling. For the Daylesford sample, this was 2.01 million years, with +/- 110,000 years
error margin.
I mention this because it indicates
that it is in
fact quite difficult to accurately determine the age of rocks!
(This
explanation is for general interest: geology students should read the
article regarding corrections for entrapped atmospheric argon, and the
hypothesis that the feldspar crystals are xeoncrysts, rather than being
formed as the lava cooled.)
The last eruptions were 5,000 years ago in Mt Gambier. Researchers estimate that the Newer Volcanics Province has
averaged one eruption every 10,800 years, so it is still considered
active.
One good source of information I found online about the New Volcanic
Province volcanos of Victoria is in this 1993 field trip guide:
Nicholls-1993
I. A. Nicholls, A.G. Greig, C.M. Gray
and R.C. Price
Newer Volcanics
Province - Basalts, Xenoliths and Megacrysts (IAVCEI Canberra
1993 Pre-Conference Field Trip Guide)
Record 3 1993/58 Australian Geological Survey Organisation.
http://www.ga.gov.au/corporate_data/14654/Rec1993_058.pdf
(Open access.)
|
This includes information about many other places of
interest,
including the Byaduk lava blisters (tumuli) formed in the lava which
flowed from what is now Mt Napier.
We have Bill Birch's book "Volcanoes of Victoria", from
the Royal Society of Victoria (there's
no web-ordering, so we called them by phone). We found this to be an excellent book.
Maps and other material can be found for searching for "Newer Volcanic"
at:
Dr Julie Ann Boyce has a number of articles which can be
downloaded at:
A compendium of some of her articles on Victorian volcanoes is in
a long PDF:
This is a description of Julie Boyce's Google Maps project: "Victorian
Volcanoes":
There's also a great article by Jeremy Bourke: "Forged by fire: Volcanoes in Victoria":
and a list of Victorian volcanos to visit, including our illustrious
Wombat Hill, which is the Daylesford Botanical Gardens, planted with
conifers from all over the world:
A website devoted to Victorian volcanoes appeared in 2017:
Back to Daylesford . . .
Despite the numerous examples of "Wombat" in local place names, we are
yet to see a wombat here. Bullarto is the closest we have seen
signs of wombats, and we are yet to see one alive. This
interactive map of wombat sightings:
indicates that this area is at about the western-most limit of wombat inhabitation in Victoria.
Gold mining was originally alluvial (gold washed from reefs into river
beds) and then went underground chasing the (quartz) reefs
themselves. I don't yet know enough about the geological history,
but it is my impression that the gold is not connected with this recent
volcanic activity - the incursion of quartz reefs is probably much
older than the last few million years.
I understand (from the abstract of http://researchonline.jcu.edu.au/1785/
"Stable
isotope geochemistry of cold CO2-bearing mineral spring waters,
Daylesford, Victoria, Australia: sources of gas and water and links
with waning volcanism" I. Cartwright et al. 2002) that the
mineral and carbon-dioxide content of the many local springs is due to
the volcanism. Although none of the springs are hot, (according
to some other reference I can't recall) their mineral composition
varies so much that the source of the mineralization and CO2 must be
only a few hundred metres below the surface.
The Daylesford area was originally settled by Europeans for farming,
but it became one of many gold-mining areas in Victoria in the 1950s (https://en.wikipedia.org/wiki/Daylesford,_Victoria).
The gold was introduced into the area in quartz reefs, which
intruded into the sedimentary rock. Some of this was eroded and
the gold tended to settle in the creek beds, due to its high
density. This alluvial gold was found first at Wombat Flat, at
the south-east corner of what is now Daylesford Lake.(See note below.)
The only proper account of the mining
history of Daylesford seems to be
this book. There's a copy of the first edition in the local
library (http://www.centralhighlandslibraries.org.au)
.
100 YEARS OF DAYLESFORD GOLD MINING
HISTORY
By HENRY T. MADDICKS
Assisted by K. H. BUTLER
This was published by the
Daylesford Historical Society - now the Daylesford & District
Historical Society http://www.daylesfordhistory.com.au/
.
The second edition of the book is now available from The Society at the
Daylesford Museum (this is not its proper title, but I guess people
will search for this term) and was launched there at 6:30PM on
9th December 2016. The Society's collection (museum)
is located at 100 Vincent St, just south
of the post office.
I guess that this book was written and published in the early
1980s. The first sentence of the author's biography is: "H.
T. Maddicks, well known in Daylesford as a 1920's Radio and later TV
Engineer, a business he ran in Daylesford for 40 years.
The last page mentions "Abco Print, Daylesford". This indicates
that it was printed and perhaps typeset (Linotype) here. Linotype
machines were still used in those days before computers enabled
phototypesetting. A few historical books printed (or perhaps
published) by this company can be found with
this
Google search.
This
library page refers to a 1981 book manufactured by "Daylesford Abco
Printing Service".
Despite giving a good account of a hundred or
more mines, there is no
discussion of the techniques used (at least in the first
edition). Did they drill and blast (with
what kind of explosives?) or did they simply hack away at the
rock. Some of the rock here is quite soft - the sedimentary rock
can crumble easily, but the volcanic and some other rocks (I guess
metamorphic) are much harder.
In another page here ../04-daylesford-not-winter/
is a picture of one of the water races which supplied the mining
industry - 240 miles of water races in total in the Daylesford area.
The 1980s original edition of this book lacks a map -
and a good companion to reading it would be this map, now freely
available as a PDF:
This covers several gold fields, including the rich and quickly mined
shallow alluvial deposits of Yandoit, to the north of Daylesford.
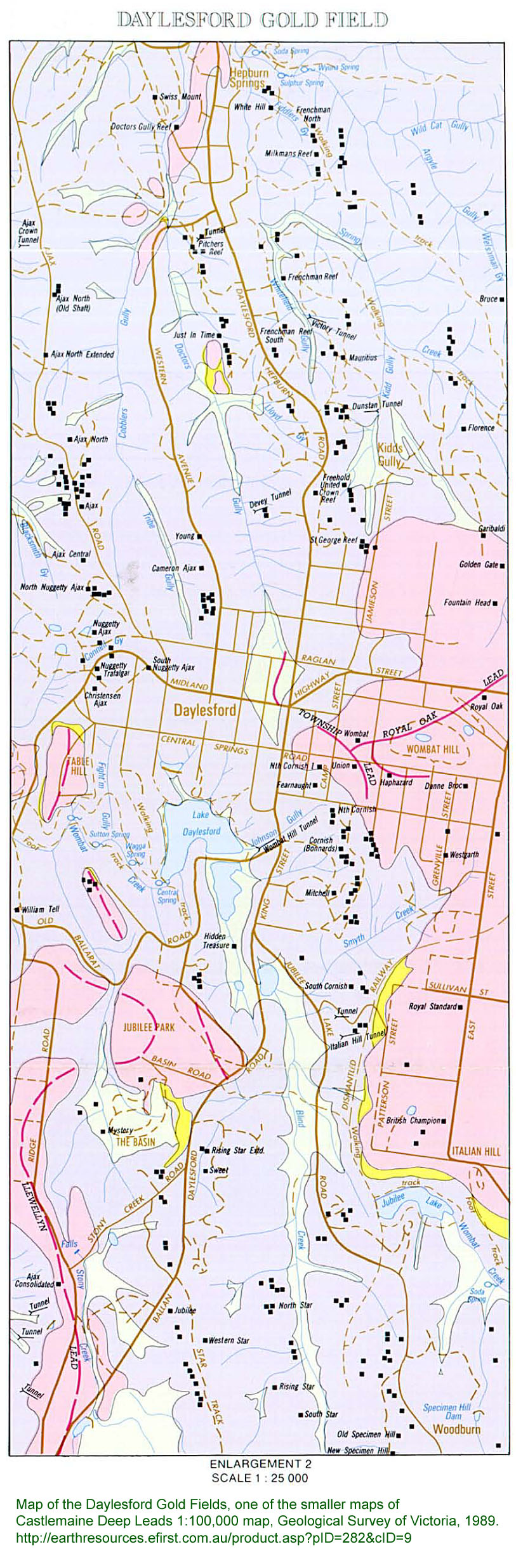
From the above, here is a smaller, more detailed, map of the Daylesford
gold fields. To aid with printing, here it is as pages 1, 2 and 3.
(More on maps and guides below.)
This book (chapter 3) mentions the exact location of the first gold
found in the Daylesford area:
The
area referred to in evidence to the Gold Rewards Board was in Wombat
Creek behind the present day Wentworth Hotel - Old Bakery area, now
covered by the head waters of Lake Daylesford.
A friend of ours is of the family who ran the bakery - they used to
deliver bread around Daylesford with five draught horses in the early
1960s. The
main industry of Daylesford in those days saw milling, though the
woolen mill in East St was also active. Here is the location:
I understand that the first gold was won from alluvial deposits (the
current lake area was heavily mined, and then used as a market garden,
before being dammed to make the lake), with tunnels chasing gold reefs,
or more frequently pursuing the creek-beds which were buried by the
volcano a few million years (I guess) ago.
The Township Lead was such a creek bed, and mining took place all the
way along it, with shafts and tunnels under the main street and under
the area where the churches are located, such as where the mine "Union"
was located.
As far as I know, the gold (in quartz infusions) long predates,
and is unrelated to, the volcanism. The volcanic activity is
associated with the rise of carbon dioxide into the water table in this
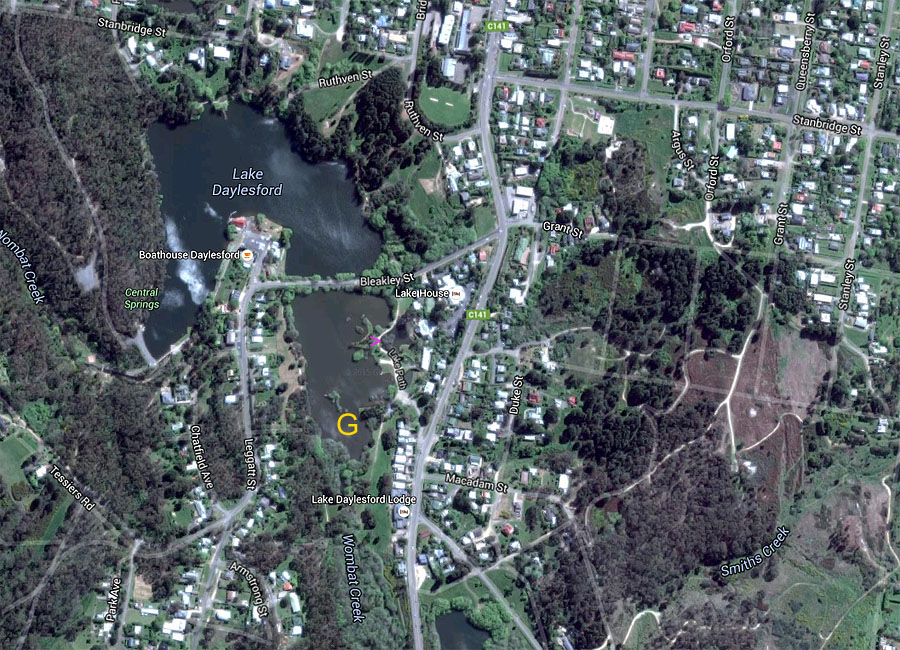
area. In some locations, such as where the pink >
points in the above image from Google Maps, gas - presumably
carbon dioxide (C02)
- can be seen continually bubbling to the surface of the water, here a
small lake to the east of the main lake. Continual bubbles can be
seen in the main lake in a line leading approximately NNW from there,
including on the other side of Bleakley St, though these are normally
only visible if there is little wind.

The other location we have seen continual bubbles is in the creek at
Tipperary Springs.
It seems that the pressure of this gas varies. Twice (between
April 2014 and October 2015) we have seen Wombat Flat spring (a few
tens of metres SW of the above location) gushing with a little water
and a lot of gas. Normally, the pump there only produces water if
the lever is pushed. This extra pressure seems to be correlated
with a little more activity in these continually bubbling spots in the
small
lake:

Here is a 15 second video, with no sound: CO2-bubbles-at-Daylesford-Lake.mpg.
Click the following image for a 15 second video (again without
sound) of Wombat Flats Spring gushing carbon dioxide on 28 September
2015:
So while Daylesford is now, in part, a place where one can come to
get one's chakras realigned, or luxuriate for a day in a spa, it was, a
few decades ago, a town of sawmills. In the century and a half
past, it was an extremely busy gold mining town, with rough, cold, wet,
living alongside multiple newspapers, breweries and entertainment
establishments with ballrooms and dance halls. The Daylesford Brass
Band traces its history to the gold mining days of 1861.
Daylesford has not gone all boutique. There is a speedway, a
concrete plant and, a few km to the east, a sheep abattoir.
There are many remnants of gold mining, and some buildings date to
those days. There are excellent bookshops - new and second-and
bookshop (Paradise
Books), and a second-hand book and cafe at the lake (Book Barn).
Trentham's bookshops include the quiet, bright and beautifully curated
Dr
B's Bookstore at 40B High St, and a second-hand bookshop (I can't
remember the name) at 19 Victoria St, between Quarry St and Station St,
just east of the lake.
See ../09-daylesford-trentham-food-etc/
for further information on local cafes and other businesses Tina and I
particularly like: .
There are no Bureau of Meteorology predictions or observations for
Daylesford
specifically - Ballarat
is the closest. However the CFA, in
Bridport St SW of Coles, has a weather station:
The CFA lists warnings and planned burns at: http://warnings.cfa.vic.gov.au/
. The 4 day forecast and current situation for fire danger for
all of Victoria is at http://www.cfa.vic.gov.au/warnings-restrictions/total-fire-bans-and-ratings/
with Daylesford in the Central district.

Daylesford, looking north-west, from the Cornish Hill lookout at
the south end of Orford St, May 2014. The cream building with
tower is the post office.

On the 26 June 2014, on a gloomier day, looking more to the
north. The Bunya Pine which was blown down in the mini-tornado of
February 2015 (see ../01-mini-tornado-feb-2015/)
is visible just behind the
spire on the left.
Here are some maps and guides which I think will be of interest to
people visiting the Daylesford and Castlemaine areas with an interest
in bushwalking and mining history (see above for the 100 Years of
Daylesford Mining History book):
The Goldfields Track Walking and
Cycling Guide. This booklet has many map pages and has full
details of the track which runs from Mt Bunninyong, south-east of
Ballarat, through Creswick, Daylesford, Hepburn Springs, Vaughn,
Fryerstown and Castlemaine to Bendigo. The book is available from
The Book Barn on Lake
Daylesford and from http://www.goldfieldstrack.com.au
.
Two maps we bought from http://www.melbmap.com.au:
Meridian Wombat State Forest 4WD
Touring Map 4th edn (link)
Hayman's Daylesford-Castlemaine-Ballarat Forest Activities Map (link)
This is one of several pages
concerning Daylesford and places nearby. The index for those
pages is here: ../.









